Epstein-Barr Virus (EBV)-Transformed B-Lymphoblastoid Cell-Lines
Reference cell-lines generated by Laboratorio de Genómica Viral y Humana.
Facultad de Medicina, Universidad Autónoma de San Luis Potosí.
The deficiency of positive control material for molecular genetic testing has been identified as
an issue of utmost urgency for clinical genetic testing. Molecular genetic testing is an
important component of routine health care as the genetic foundations of a growing
number of diseases are revealed. In addition studies of human population genetics and diversity
have suffered from the lack of suitable, well-characterized biological specimens. The availability
of positive control material for performance evaluation and quality assurance of existing tests
and molecular assays as well as for development and validation of novel tests, is critical to the
genetic testing community. Reliable, high-quality control material for genetic testing can be
provided by stably transformed B-lymphocyte cell lines derived from blood from reference subjects
or those bearing genetic, ethnical or clinical features of interest. Lymphoblastoid cell lines are
used extensively by proficiency testing groups, and many cell lines of this type are available
in our repository.
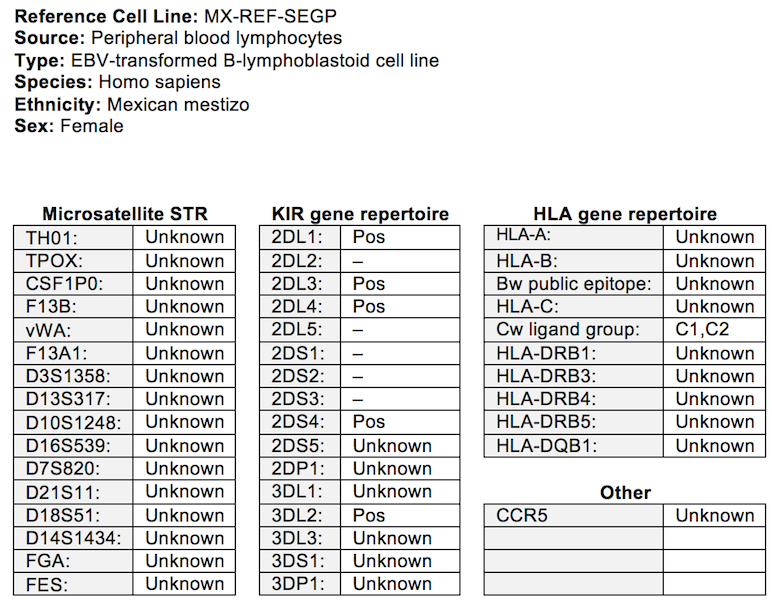
MX-REF-SEGP (SEGP)
Epstein-Barr Virus (EBV)-transformed B-Lymphoblastoid cell line generated from peripheral
blood cells isolated from a Mexican mestizo donor as a source of reference genomic DNA
for molecular method optimizations.
Day +11 after transformation, 4x amplification
Day +11 after transformation, 10x amplification
Day +11 after transformation, 25x amplification, phase contrast